EBV and NPC carcinogenesis—an alternative viewpoint
The etiology of nasopharyngeal carcinoma (NPC) has been causally linked to environmental and ethnic factors. In this article, we aim to put forward a proposed mechanism linking Epstein Barr Virus (EBV) infection to the observed ethnic and geographical distributions of this unique disease.
Primary nasopharynx epithelial cells show very low EBV infection efficiency. A pertinent question in the etiology of NPC would be how and when EBV enters the host cell in NPC patients. Studies on the mouse gamma herpesvirus 68 suggest there is a temporal relationship between the period in life that infection occurs and the subsequent disease that develops. Infection occurring during the neonatal period of the mouse results in respiratory epithelial infection, whereas infection in the adult mouse results in an “infectious mononucleosis” like syndrome (1). Similarly, in humans, EBV infection appear to occur early in life in the susceptible populations for NPC (Southern China, Southeast Asia); whereas in populations with low incidence of NPC (such as in Caucasians), EBV infections occur later in the teens. Incidentally however, this later onset of EBV infection appears to be associated with a higher incidence and a bimodal peak of EBV-related Hodgkin’s Lymphoma in these populations. Of further note, in cities like Hong Kong and Singapore, where the incidence of NPC has been in decline in the last few decades, the incidence of Hodgkin’s Lymphoma in those 2 cities is slowly picking up and beginning to mimic the bimodal peak found in the Caucasian countries (2). Other infection-related cancers like Hepatitis B-associated hepatocellular carcinoma (HCC), H. pylori related gastric carcinoma (GC) and even Merkel cell polyomavirus related Merkel Cell Carcinomas have in common either perinatal, neonatal or early infections. We put forward that a similar mechanism may be at play here, and suggest a postulated mechanism for how EBV might enter the epithelial cell during the neonatal period, giving rise to a chain of events that ultimately results in the carcinogenesis of NPC.
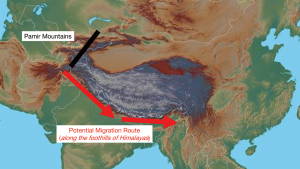
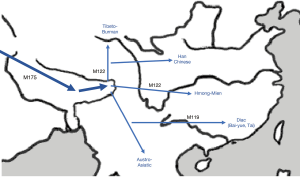
Periods of severe aridity between 135,000 and 75,000 years ago in East Africa are believed to be the impetus for human migration out of Africa (3). By about 50,000 to 40,000 years before present (ybp), some were thought to have arrived in Central Asia and settled there. Changes in climatic conditions with the glacial maximum made life difficult again and caused further dispersal. To the east were the insurmountable Pamir and Himalayan mountain ranges, so this forced those who moved east to skirt along the foothills of the Himalayas (in the narrow corridor of present-day Nepal), as they migrated in search of more hospitable environments (Figure 1). But these foothills were also holo-endemic for malaria (right until the time of the British, when they eliminated malaria) (4). This malady is particularly fatal amongst children, who are prone to developing cerebral malaria—and the cause of death is not from the parasite itself, but the immune response to it. We speculate that perhaps malaria selected for a phenotype that had an attenuated immune response to cerebral malaria. Toll like receptor (TLR)-8 which has unique polymorphisms found only in East Asians (5), is a frontrunner. It is the most selected of all the TLRs (6), and is also the only TLR, which is mature during the neonatal period (7). In mice (which do not have a functioning TLR8), loss of TLR7 (which is very similar to TLR8) alters cytokine production and protects against cerebral malaria (8). For those who survived this malaria bottleneck, they emerged from the area in current Northeast India into East Asia (9,10) (Figure 2), bringing with them the new TLR8 polymorphism that had enabled them to survive cerebral malaria, and they became the East Asians (or the so-called Mongoloid peoples, as described by the anthropologist Alfred Louis Kroeber (11). This new genetic polymorphism, while protective for cerebral malaria, now made their neonates susceptible to new infections like EBV, hepatitis B and even H. pylori, and subsequently NPC, HCC and GC. Of note, all 3 cancers are uniquely prevalent amongst East Asians. The East Asian TLR8-rs3764879 polymorphism is also associated with hepatitis C virus infection, and susceptibility to tuberculosis and periodontitis (12-14). Intriguingly, TLR8 is located on the X-chromosome, and NPC, HCC and gastric cancers all have a 3:1 male sex preponderance, suggesting a possible sex-linked recessive form of inheritance. Hu and colleagues typing 247 cases of NPC concluded that there was strong supporting evidence for the existence of a southern Chinese specific, recessive NPC gene closely linked to the HLA region as a major determinant of the Chinese risk for the disease (15), and thus, giving TLR8 greater credence for its potential involvement in the pathogenesis of NPC.
Besides TLR8, the EDAR gene has also unique polymorphisms found only in East Asians. The EDARV370A is responsible for the unique hair, facial and dental features of East Asians, and also for more densely branched mammary glands and an increased number of eccrine or sweat glands (16). This EDAR allele was thought to have arisen in East Asia about 30,000 years ago, but the cause of this selection is unknown. One speculation is that this variant affects NF-kB activity. Brik and colleagues from Germany (17) using an in vitro transfection assay, demonstrated that 370A enhances NF-kB activity. On the other hand, Fujimoto et al from Japan (18) showed that the C allele associated with thicker hair showed lower relative luciferase activities than the T allele in cell line experiments, and suggest that amino acid replacement in the death domain causes a functional change and results in the lower activity of NF-kB. Furthermore, they argue that steroids induce NF-kB suppression, as well as hair regrowth, which supports that lower NF-kB level may be associated with higher activity of hair formation. Could this lower NF-kB activity also have reduced the severity of cerebral malaria? (19,20).
There are at least 2 potential effects of the EDAR gene polymorphism that might have an effect on the EBV-NPC carcinogenesis cascade. Ectodysplasin and Wnt pathways are required for salivary gland morphogenesis (21) and a previous study reports that the histological structures of sebaceous glands of East Asians appear more juvenile compared with those of adult White or African Americans (22). Mouse studies suggest a significant difference in glandular structure between adult Edar transgenic and wild type animals, with a greater degree of epithelial branching in the transgenic glands. The mammary glands are formed in a manner similar to that of the salivary glands by the repeated branching of an ingrowing epithelial cord. Homozygous transgenic animals with elevated Edar signaling displayed a clear elaboration of mammary structure relative to wild type, with both greater extent of epithelial penetration into the fat pad and a greater density of epithelial branching. Morphological changes of a direction similar to those reported here in the mouse are likely to occur in the glands of humans expressing EDAR370A. This suggests that rs3827760 may contribute to phenotypic variation in glandular structure and function among modern human populations. For example, among women living in the United States, mammographic breast density is reported to be greater in those with Chinese and Japanese ancestry than in those of European and African descent, consistent with the observation of increased epithelial density in Edar transgenic mice (23). Does this greater density of the glands signify perhaps a wider surface area for EBV to enter an epithelial cell?
Secondly, EDAR is also responsible for the dental traits in East Asians making them more susceptible to chronic periodontitis (24,25)—and this chronic infection may also play a role in promoting the carcinogenesis cascade (26-29).
Human leukocyte antigen (HLA) is involved in antigen presentation to CD8+ T cells, natural killer cells (NK cells), and CD4+ T cells (30). While the precise genetic risk factors still remain largely unknown, HLA genes have been a focus of attention, since the discovery of an HLA association with NPC in 1973. Genetic differences between Northeast Asian (NEA) and Southeast Asian (SEA) populations have been observed in numerous studies. Sanchez-Mazas (31) using HLA data observed a genetic differentiation between NEA and SEA populations following a continuous pattern from north to south, and they show a significant and continuous decrease of HLA diversity in the same direction. This continuity is shaped by clinal distributions of many HLA lineages and alleles with increasing or decreasing frequencies along the latitude. These results bring new evidence in favor of the “overlapping model” proposed previously for East Asian peopling history, whereby modern humans migrated eastward from western Eurasia via two independent routes along each side of the Himalayas and, later, overlapped in East Asia across open land areas. The HLA genes associated with NPC are particularly prevalent in SEA populations in Southern China—the epicentre for NPC.
The “4.2-kiloyear acidification event” (32), one of the most severe climatic events resulting in droughts and severe floods, occurred around 2200BC and has been hypothesized to have caused the collapse of several ancient civilizations including the Old Kingdom in Egypt, the Indus Valley Civilization and the Lianzhu culture in the lower Yangtze River area. It is also believed to have precipitated the Austronesian expansion, which brought the Mainland Southeast Asians into Islandic Southeast Asia and subsequently eastwards to Polynesia and westwards to Madagascar, and with it they also brought along with them NPC (10,33).
Thus, in Southeast Asians we see the convergence of a perfect storm—after infection, the virus benefits from an attenuated innate immune response (TLR8) during the neonatal period and this is followed by a possibly defective cell-mediated immunity (HLA), allowing the viral infection to take hold. But this still does not explain how the virus gains entry into the epithelial cell. Hutt-Fletcher (34) suggests the role of integrin αvβ6 for viral entry into epithelial cells. The αvβ6 is expressed during fetal development, but in adults is expressed only at low levels on most normal epithelial tissues in vivo, and its expression is up-regulated only during tissue remodeling, accompanying wound-healing and inflammation. Breuss (35) in a study of β6 expression in baboons found that the newborn primates still harboured residual β6 expression. This together with TLR8 being the only mature TLR during the neonatal period may then explain the importance of neonatal infection for NPC carcinogenesis to occur.
NPC is considered to be an epithelial tumour characterised by expression of CK5/6, p63 and p40. The fossa of Rosenmuller has a transformation epithelial zone, which is active only in the first 10 years of life (36), and is thought to be the source of the progenitor EBV-infected epithelial cells, which transform into a cancer. To pathologists, lymphoepitheliomas are histologically identical (in morphology and histochemical studies) irrespective of their site of origin—whether from the post nasal space or the parotid glands (37). This then begs the question—if NPC is thought to arise from the epithelial cells of the transformation zone, then where is this epithelium in the parotid glands of Eskimomas? In a similar vein, NPC-in-situ is a rare entity unlike Cervical Intraepithelial Neoplasia (CIN) which is thought to have arisen from a transformation zone in the cervix (38). NPC is also known to spread through the submucosa (39). A very recent Dutch study (40) suggesting the presence of a “new” tubarial salivary gland in the torus tubarius casts new light on this problem. Perhaps NPC is not an epithelial tumour arising from the epithelium, but rather a salivary gland tumour instead. This would bring it in line with all the other non-NPC lymphoepitheliomas. In a recent study, Boecker and colleagues (41) showed the presence of P63/K5+ progenitor cells residing in the basal portion of excretory and striated ducts of the salivary glands; and giving rise to both neoplasia as well as non-neoplastic squamous metaplasia that differentiate toward K10/13+ squamous cells. We also know that the salivary glands are another source for the lifelong persistence of EBV (42). In East Asians, we alluded to previously that the EDAR gene has rendered an increase in epithelial density of their salivary glands. Conceivably, a neonate infected with EBV will have his salivary gland bathing in EBV, some of which might attack a progenitor epithelial cell; and with no or an attenuated response from the host immune system, allow the cascade towards cancer to proceed.
Daud (43) in a study of Kenyan mothers showed the presence of potentially infectious EBV in their breast milk and suggests this as a potential source of infection for the Kenyan babies. There is no reason to believe that this would not be so in East Asians (44). It is interesting to note that in the more developed countries in East Asia, it was the advent of evaporated milk formulas in the 1930s, as well as industrialisation in the 1950s (which resulted in the rise of a significant female workforce) (45,46), that led to a reduction in breast-feeding (47,48), which could perhaps account partially for the declining incidence of early EBV infections (and NPC incidence) alluded to earlier. It would be interesting to speculate if the re-introduction and encouragement of breast-feeding would reverse this trend.
In conclusion, we present an alternative narrative of the early phases of NPC carcinogenesis based on historical, anthropological and real-world observations. Starting first around 30,000 years ago, when the first proto-East Asians migrated into East Asia, they acquired a genetic mutation to survive malaria, only to find themselves now susceptible to new pathogens, and this together with a further susceptibility for chronic infections completes all the ingredients needed for the creation of NPC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Maria Li Lung, Lawrence S. Young) for the series “NPC Biomarkers” published in Annals of Nasopharynx Cancer. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/anpc-21-1). The special series “NPC Biomarkers” was commissioned by the editorial office without any funding or sponsorship. J. Wee serves as an unpaid editorial board member of Annals of Nasopharynx Cancer from Jan 2021 to Dec 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ptaschinski C, Rochford R. Infection of Neonates With Murine Gammaherpesvirus 68 Results in Enhanced Viral Persistence in Lungs and Absence of Infectious Mononucleosis Syndrome. J Gen Virol 2008;89:1114-21. [Crossref] [PubMed]
- Hjalgrim H, Seow A, Rostgaard K, et al. Changing Patterns of Hodgkin Lymphoma Incidence in Singapore. Int J Cancer 2008;123:716-9. [Crossref] [PubMed]
- Tattersall I. Out of Africa: modern human origins special feature: human origins: out of Africa. Proc Natl Acad Sci U S A 2009;106:16018-21. [Crossref] [PubMed]
- Modiano G, Morpurgo G, Terrenato L, et al. Protection against malaria morbidity: near-fixation of the alpha-thalassemia gene in a Nepalese population. Am J Hum Genet 1991;48:390-7. [PubMed]
- Cheng PL, Eng HL, Chou MH, et al. Genetic polymorphisms of viral infection-associated Toll-like receptors in Chinese population Transl Res 2007;150:311-8. [Crossref] [PubMed]
- Barreiro LB, Ben-Ali M, Quach H, et al. Evolutionary dynamics of human Toll-like receptors and their different contributions to host defense. PLoS Genet 2009;5:e1000562 [Crossref] [PubMed]
- Levy O, Suter EE, Miller RL, et al. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 2006;108:1284-90. [Crossref] [PubMed]
- Baccarella A, Huang BW, Fontana MF, et al. Loss of Toll-like receptor 7 alters cytokine production and protects against experimental cerebral malaria. Malar J 2014;13:354. [Crossref] [PubMed]
- Blench R. ‘The world turned upside down’: sago-palm processors in northeast India and the origins of Chinese civilization. In: Helen Lewis (ed). Oxford, UK: EurASEAA14 Volume I: Ancient and Living Traditions: Papers from the Fourteenth International Conference of the European Association of Southeast Asian Archaeologists. Archaeopress Access Archaeology, 2020.
- Wee JT, Ha TC, Loong SL, et al. Is nasopharyngeal cancer really a "Cantonese cancer"? Chin J Cancer 2010;29:517-26. [Crossref] [PubMed]
- Kroeber AL. Anthropology: race, language, culture, psychology, pre-history. New York: Harcourt, Brace and Company, 1948.
- Wang CH, Eng HL, Lin KH, et al. Functional polymorphisms of TLR8 are associated with hepatitis C virus infection. Immunology 2014;141:540-8. [Crossref] [PubMed]
- Zhou Y, Zhang M. Associations between genetic polymorphisms of TLRs and susceptibility to tuberculosis: A meta-analysis. Innate Immun 2020;26:75-83. [Crossref] [PubMed]
- Leite FRM, Enevold C, Bendtzen K, et al. Pattern recognition receptor polymorphisms in early periodontitis. J Periodontol 2019;90:647-54. [Crossref] [PubMed]
- Hu SP, Day NE, Li DR, et al. Further evidence for an HLA-related recessive mutation in nasopharyngeal carcinoma among the Chinese. Br J Cancer 2005;92:967-70. [Crossref] [PubMed]
- Kamberov YG, Wang S, Tan J, et al. Modeling recent human evolution in mice by expression of a selected EDAR variant. Cell 2013;152:691-702. [Crossref] [PubMed]
- Bryk J, Hardouin E, Pugach I, et al. Positive selection in East Asians for an EDAR allele that enhances NF-kappaB activation. PLoS One 2008;3:e2209 [Crossref] [PubMed]
- Fujimoto A, Kimura R, Ohashi J, et al. A scan for genetic determinants of human hair morphology: EDAR is associated with Asian hair thickness. Hum Mol Genet 2008;17:835-43. [Crossref] [PubMed]
- Labbé K, Miu J, Yeretssian G, et al. Caspase-12 dampens the immune response to malaria independently of the inflammasome by targeting NF-kappaB signaling. J Immunol 2010;185:5495-502. [Crossref] [PubMed]
- Tripathi AK, Sha W, Shulaev V, et al. Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood 2009;114:4243-52. [Crossref] [PubMed]
- Häärä O, Fujimori S, Schmidt-Ullrich R, et al. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development 2011;138:2681-91. [Crossref] [PubMed]
- Kallapravit B. Racial, age and regional differences in human sebaceous glands of the head and neck. Am J Phys Anthropol 1963;21:121-33. [Crossref] [PubMed]
- Chang SH, Jobling S, Brennan K, et al. Enhanced Edar signalling has pleiotropic effects on craniofacial and cutaneous glands. PLoS One 2009;4:e7591 [Crossref] [PubMed]
- Lee SA, Kim AC, Prusa LA Jr, et al. Characterization of dental anatomy and gingival biotype in Asian populations. J Calif Dent Assoc 2013;41:31-3, 36-9. [PubMed]
- Matsson L, Sjödin B, Blomquist HK. Periodontal health in adopted children of Asian origin living in Sweden. Swed Dent J 1997;21:177-84. [PubMed]
- Liu Z, Chang ET, Liu Q, et al. Oral Hygiene and Risk of Nasopharyngeal Carcinoma-A Population-Based Case-Control Study in China. Cancer Epidemiol Biomarkers Prev 2016;25:1201-7. [Crossref] [PubMed]
- Wen BW, Tsai CS, Lin CL, et al. Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM 2014;107:283-90. [Crossref] [PubMed]
- Rickinson AB. Co-infections, inflammation and oncogenesis: future directions for EBV research. Semin Cancer Biol 2014;26:99-115. [Crossref] [PubMed]
- Jacqueline C, Tasiemski A, Sorci G, et al. Infections and cancer: the “fifty shades of immunity” hypothesis. BMC Cancer 2017;17:257. [Crossref] [PubMed]
- Simons MJ. Nasopharyngeal carcinoma as a paradigm of cancer genetics. Chin J Cancer 2011;30:79-84. [Crossref] [PubMed]
- Di D, Sanchez-Mazas A. HLA variation reveals genetic continuity rather than population group structure in East Asia. Immunogenetics 2014;66:153-60. [Crossref] [PubMed]
- 4.2-kiloyear event. Wikipedia article. Available online: https://en.wikipedia.org/wiki/4.2-kiloyear_event
- Trejaut J, Lee CL, Yen JC, et al. Ancient migration routes of Austronesian-speaking populations in oceanic Southeast Asia and Melanesia might mimic the spread of nasopharyngeal carcinoma. Chin J Cancer 2011;30:96-105. [Crossref] [PubMed]
- Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc Natl Acad Sci U S A 2009;106:20464-9. [Crossref] [PubMed]
- Breuss JM, Gallo J, DeLisser HM, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995;108:2241-51. [Crossref] [PubMed]
- Ali MY. Histology of the human nasopharyngeal mucosa. J Anat 1965;99:657-72. [PubMed]
- He Q, Zhou Y, Fu C, et al. Lymphoepithelioma is a nonkeratinizing squamous cell carcinoma with Epstein-Barr virus infection in China. J Cancer Res Ther 2017;13:807-12. [Crossref] [PubMed]
- Pak MW, To KF, Lo YM, et al. Nasopharyngeal carcinoma in situ (NPCIS)--pathologic and clinical perspectives. Head Neck 2002;24:989-95. [Crossref] [PubMed]
- Sham JS, Wei WI, Kwan WH, et al. Fiberoptic endoscopic examination and biopsy in determining the extent of nasopharyngeal carcinoma. Cancer 1989;64:1838-42. [Crossref] [PubMed]
- Valstar MH, de Bakker BS, Steenbakkers RJHM, et al. The tubarial salivary glands: A potential new organ at risk for radiotherapy. Radiother Oncol 2021;154:292-8. [Crossref] [PubMed]
- Boecker W, Stenman G, Loening T, et al. Squamous/epidermoid differentiation in normal breast and salivary gland tissues and their corresponding tumors originate from p63/K5/14-positive progenitor cells. Virchows Arch 2015;466:21-36. [Crossref] [PubMed]
- Morgan DG, Niederman JC, Miller G, et al. Site of Epstein-Barr virus replication in the oropharynx. Lancet 1979;2:1154-7. [Crossref] [PubMed]
- Daud II, Coleman CB, Smith NA, et al. Breast Milk as a Potential Source of Epstein-Barr Virus Transmission Among Infants Living in a Malaria-Endemic Region of Kenya. J Infect Dis 2015;212:1735-42. [Crossref] [PubMed]
- Glenn WK, Whitaker NJ, Lawson JS. High risk human papillomavirus and Epstein Barr virus in human breast milk. BMC Res Notes 2012;5:477. [Crossref] [PubMed]
- Leeming Frank. The Earlier Industrialization of Hong Kong. Modern Asian Studies 1975;9:337-42. [Crossref]
The World Bank in Singapore - Koo LC, Wong VC, Ho CY. Factors affecting breast-feeding among Hong Kong Chinese. Asia Oceania J Obstet Gynaecol 1986;12:469-77. [Crossref] [PubMed]
- Chua S, Viegas OA, Ratnam SS. Three decades of breast-feeding trends in Singapore. Asia Pac Popul J 1990;5:125-34. [Crossref] [PubMed]
Cite this article as: Poh SS, Wee JTS. EBV and NPC carcinogenesis—an alternative viewpoint. Ann Nasopharynx Cancer 2021;5:4.